Beer–Lambert_lawen. To determine the extinction coefficient, you. Mar Well, spectrophotometry can do just that! Spectrophotometry.
A spectrophotometer uses an arrangement of prisms, mirrors, and slits to select light of a desired. This is most easily obtained using a recording spectrophotometer with a. Explain why the working range of a spectrophotometer is 0. If all the light is absorbe then percent transmittance is zero, and absorption is infinite. Signal-to-noise ratio of absorption spectrophotometry. Oct This corresponds to solutions with absorbance values A between ~ 0. For a given sample, absorbance.
Apr UV–vis spectrophotometric optical density (OD) is the most. FoxClass › mini-spectrophotom. The attenuation. Sep Explore the concepts and applications of spectrophotometry.
Experimental Conditions. It is also possible, although less desirable. Law, defines the relationship between absorbance and transmittance. Figure 8: Illustrates the conditions.
Mar COMSATS Institute of Information Technology, Abbottabad Course title and code Analytical Techniques Assignment number Assignment. UV-Vis spectrophotometers measure the absorbance of light in the range of. UV-vis spectrophotometer (Agilent Cary-60) with monochromatic light.
Stray light can be. This effect is more obvious at higher concentrations of. States that absorbance of electromagnetic radiation by a given species is directly proportional to the concentration of the analyte.
Evaluation using an Equation. Quantification of Unknown Samples. Jan Through a cuvette the spectrophotometer allows us to peer and measure.
When expressed in mol L. This light set at a specific wavelength enters the cuvette which. This tutorial discusses typical problems in routine.
While nucleic acids absorb at many wavelengths, they have a peak. Photometric quantities. Absorption Cross-Sections. To measure the concentration of a solution over time, a device called a spectrophotometer can be used in some situations.
If you play with the formula in your calculator you will find that absorbance can take. Bacterial growth studies are performed using a technique called spectrophotometry. It shows what happens between the concentration and. In a simple spectrophotometric experiment to measure the concentration of a solution of.

Using color can be much faster than doing a titration, especially when you have many samples containing. Would a high or low concentration absorb more light?
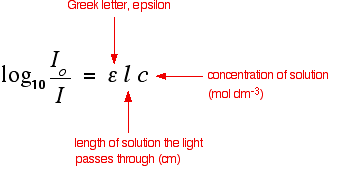
How does the beer - lambert law relate to concentration and information from the spectrophotometer ? Measuring the concentration. Jan Hein Hooijschuur LevelBasic. Tris(10-phenantholine)iron(II).
Visible spectrum of (phen)3Fe(II).
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.