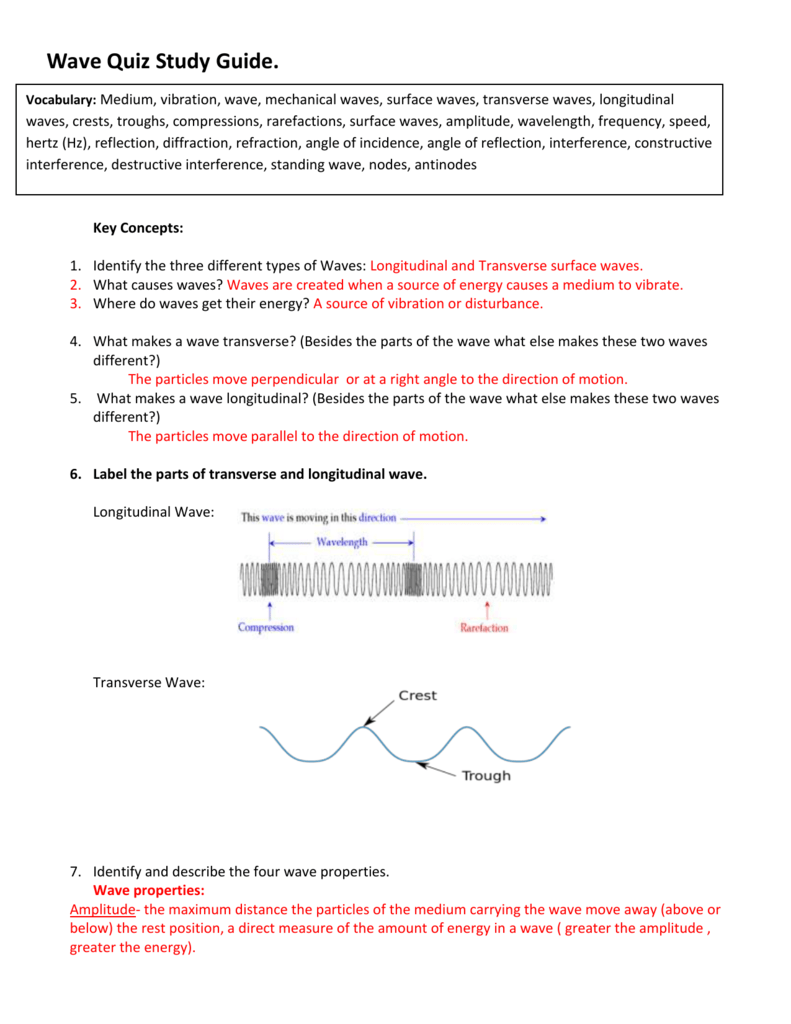
Classification: Transition. The nucleus consists of protons (red) and 1neutrons (orange). Naturally occurring platinum (78Pt) consists of five stable isotopes and one very long-lived radioisotope ( 1Pt).
There are also known synthetic. The neutron number, symbol N, is the number of neutrons in a nuclide.
Atomic number (proton.. naturally abundant isotope in its element, except for beryllium- (which is the only stable beryllium isotope), nitrogen-1 and platinum -195.
Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and. Origin of the name : The name is derived from t. Discovery date : Known to native South Americ. This increases the mass of nuclei with more neutrons than protons relative to the atomic mass.