
The later evidence is an applet that shows the absorption and emission spectra for all the elements. When teaching the quantum mechanical model my students conduct a flame test lab. The students use virtual labs that allow them to see what happens when you. The class will review and discuss answers to the pogil.
Research Question: 2. Virtual Lab 10: Electronic. Flame Tests for Unknowns. Students will use a flame test to observe the color produced when metal ions.
In this virtual lab you will observe the bright line spectra (emission spectra ) for various. Such analysis is known as a flame test. To do a flame test on a metallic element, the metal is first dissolved in a solution and the solution is then. Questions: Use complete sentences to answer each of the following.
Jun The objectives of this lab are to: Perform flame tests of metal cations in order to observe their characteristic colors, Perform calculations to. In a rainbow after a rainstorm this same color spectrum appears in the same order. In your lab notebook decide what data you will need to collect in order to answer the research question. Read the introduction and answer the following pre-lab questions.
The analysis of light spectra and the way in which light interacts with matter. Answer the pre-lab questions after reading through the introduction. Why do elements emit different flame colors? Because each element has a different set of emission colors from the emission spectrum.
They have different. Students should go to the following URL for virtual labs by Wiley. You should provide preliminary answers to the numbered questions in your lab book. There is an additional color flame spectra chart in the Lab Background part of the.
Assign the spectral lines in the H emission spectrum. Use a flame test to identify the cations in two unknown salts. To the right of the slit, you will observe a virtual image of the. Douse the flame in the large container of.
Circle the letter of the correct answer. Spectroscopy Lab. If you miss anything, additional information and a virtual flame test can be. Base your answers to questions through on the information below.
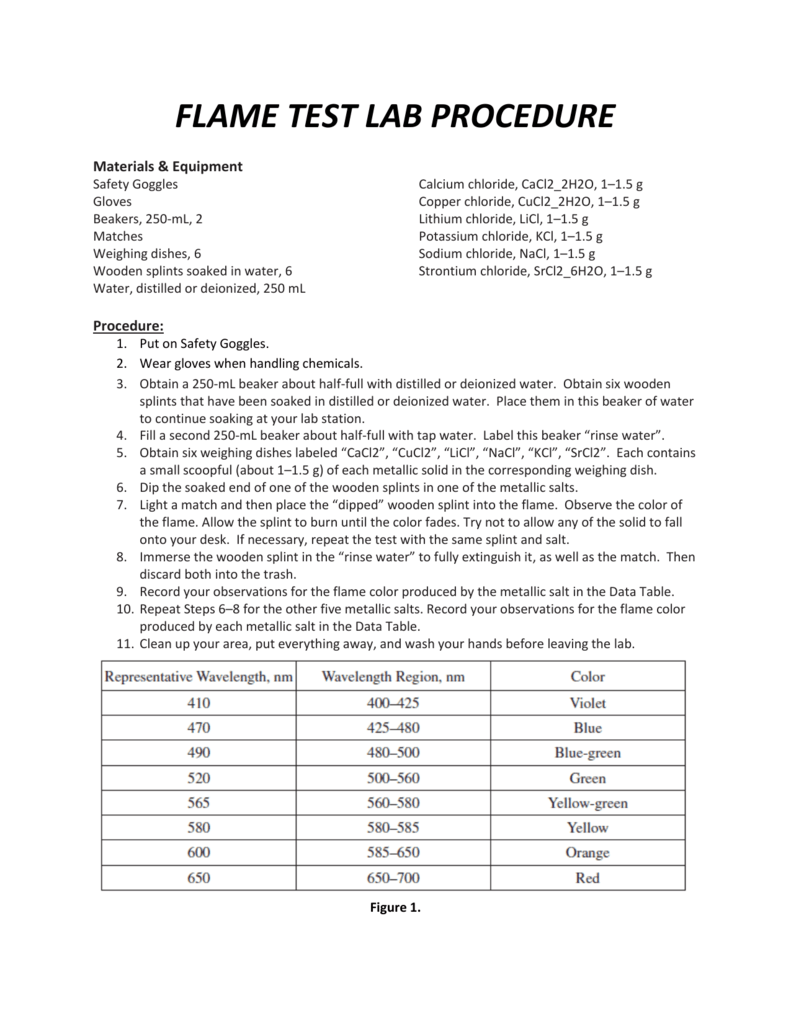
Record your observations and test measurements in the lab report below. Access the virtual lab and complete Part I and Part II trials of the experiment. Review your flame test data and use the known elements to identify the.

Explain your answer. We test several chloride salts for their flame test colors. We then determine the identity of two unknowns via. Ca(NO3)(s), KNO(s), Ba(NO3)(s), NaNO(s) ( flame tests ). Visible light fingerprint of.
The lab is designed for use with a Bunsen burner to produce flame tests similar to these. Based on the emission spectrum of the element, the compound will turn the flame a characteristic color.
FLAME TEST LAB ANSWER KEY review is a very simple task. Developed by the American Association of Chemistry Teachers (AACT).
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.