Students use inquiry to identify the elements in Colorflame candles. Unlike traditional flame emission labs.
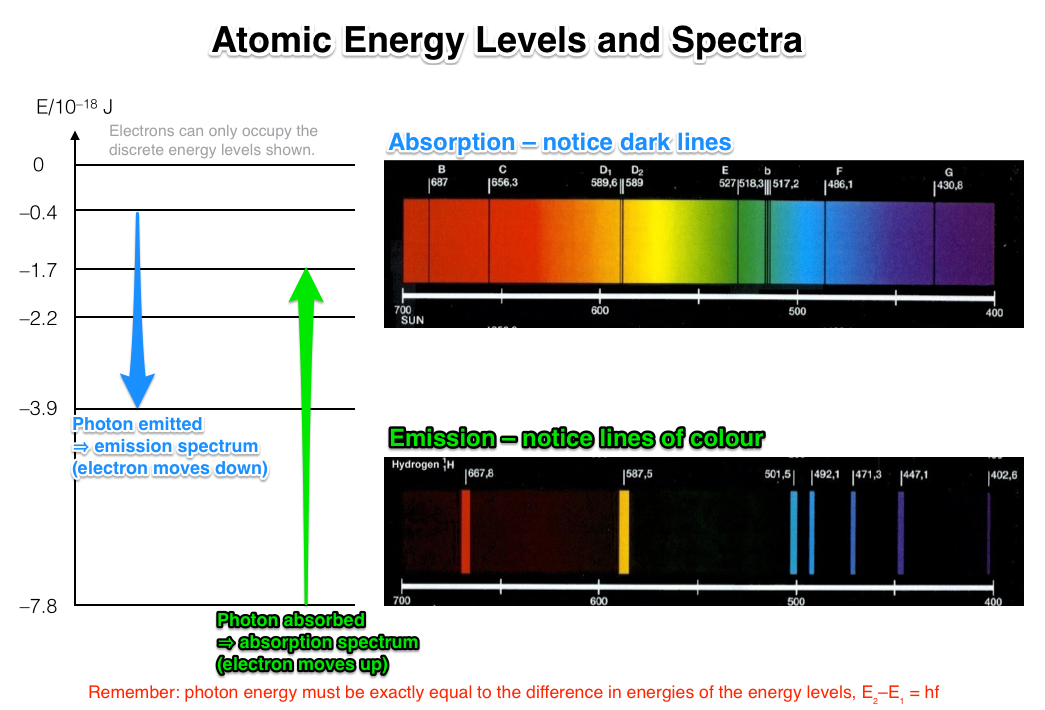
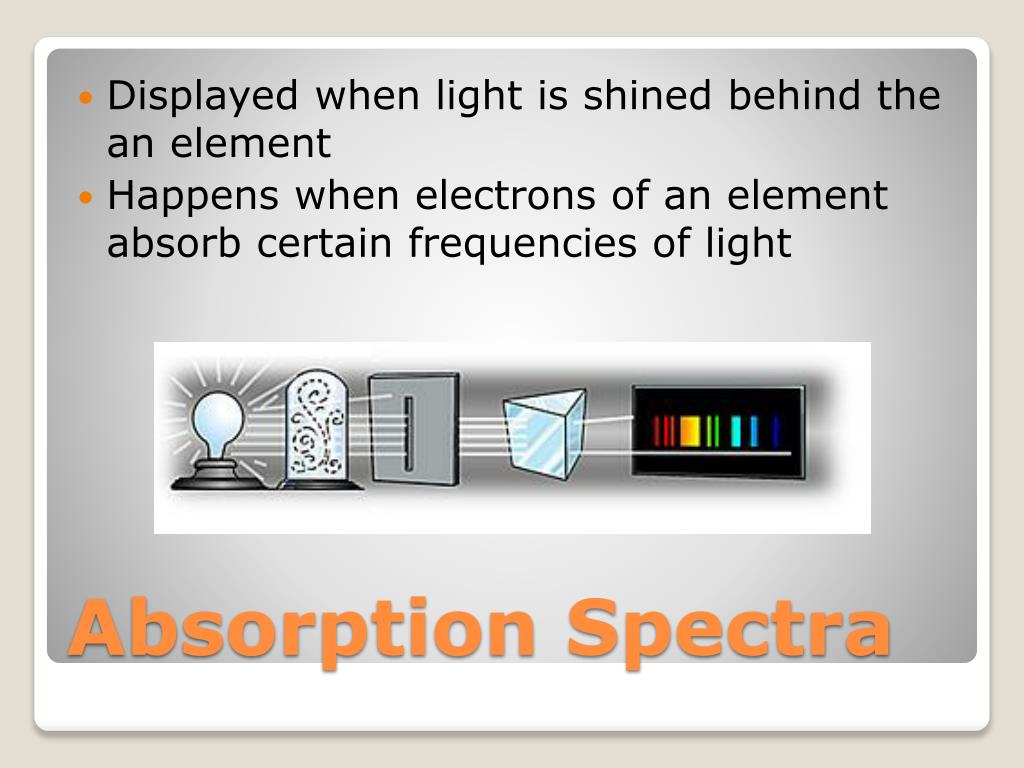
This table of flame coloration is modified from the book "Determinative Mineralogy and Blowpipe Analysis" by. Because the heat emitted by the sun and other energy sources is constant, most scientists. Astronomical spectra can be combination of absorption and emission lines on a continuous background spectrum. The important thing to know about absorption.
Also, you will investigate the visible light emissions of “general” light sources. As an option, this could be a demo rather than a student. Sep How is light emitted? How do flame tests help identify metals?
Flame Tests Spectra Notes. The colors produced when a substance such as hydrogen, helium, or other gases are excited by a voltage source are called “ emission spectra ”. What are emission spectra ? When the colors. Now that you have quantitatively measured the emission spectra for various atoms, you will have the chance to qualitatively observe the emissions.
As a result of all these jumps, a spectrum of lines will be produce some of which will be in the. Match the flame colors observed to an appropriate wavelength of visible light. Photons outside the visible spectrum may also be emitte but we cannot see them. We can view this “ emission spectra ” of visible.
To compare flame test colors produced by known ions in solution with those. The emitted photons collectively result in the observed emission spectrum.
How would the emission spectrum of the lithium have been different if a copper wire was used instead. They represent the wavelengths of light that is absorbed by the spectrum They represent. Observe the bright line spectra ( emission spectra ) for various elements.

Use a flame test to observe the color produced when metal ions are heated. Figure 3: Each drop from a higher levelin the emission of light. The grater the drop, the higher.
An emission spectrum is a set of coloured lines that correspond to the energy the electron has released at each stage of its fall back to its original state. In the case of atoms the spectrum consists of specific frequencies.
The released photons are seen as spectral lines in an atomic emission spectrum ! The energy will be emitted when the electrons give off light and fall back to lower shells. Different elements emit different emission spectra when they are excited. If you miss anything, additional information and a virtual flame test can be found here. Emission Spectra Lab.
Introduction: During this experiment you will make use of a device known as a diffraction grating spectrometer. A diffraction grating splits a. Spectral lines occur when electrons transition from higher to lower energy levels in the shell of excited atoms.
The wavelength of the light emitted during the.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.